Article
Would Stratospheric Aerosol Injection Add to Acid Rain?
The sulphate particles that could be used for stratospheric aerosol injection (SAI) would add to acid rain. With sulphate pollution from burning fossil fuels on the decline, how much could SAI slow progress in reducing acid rain?
Key takeaways
- Acid rain occurs when certain pollutants, such as sulphur dioxide (SO2), react in the atmosphere to form acidic products.
- Acid rain can negatively affect ecosystems by increasing the acidity of soils and inland water systems; it is most severe near industrial regions with weaker air pollution regulations.
- SAI using sulphates could slow progress on reducing acid rain and change how it is distributed around the world.
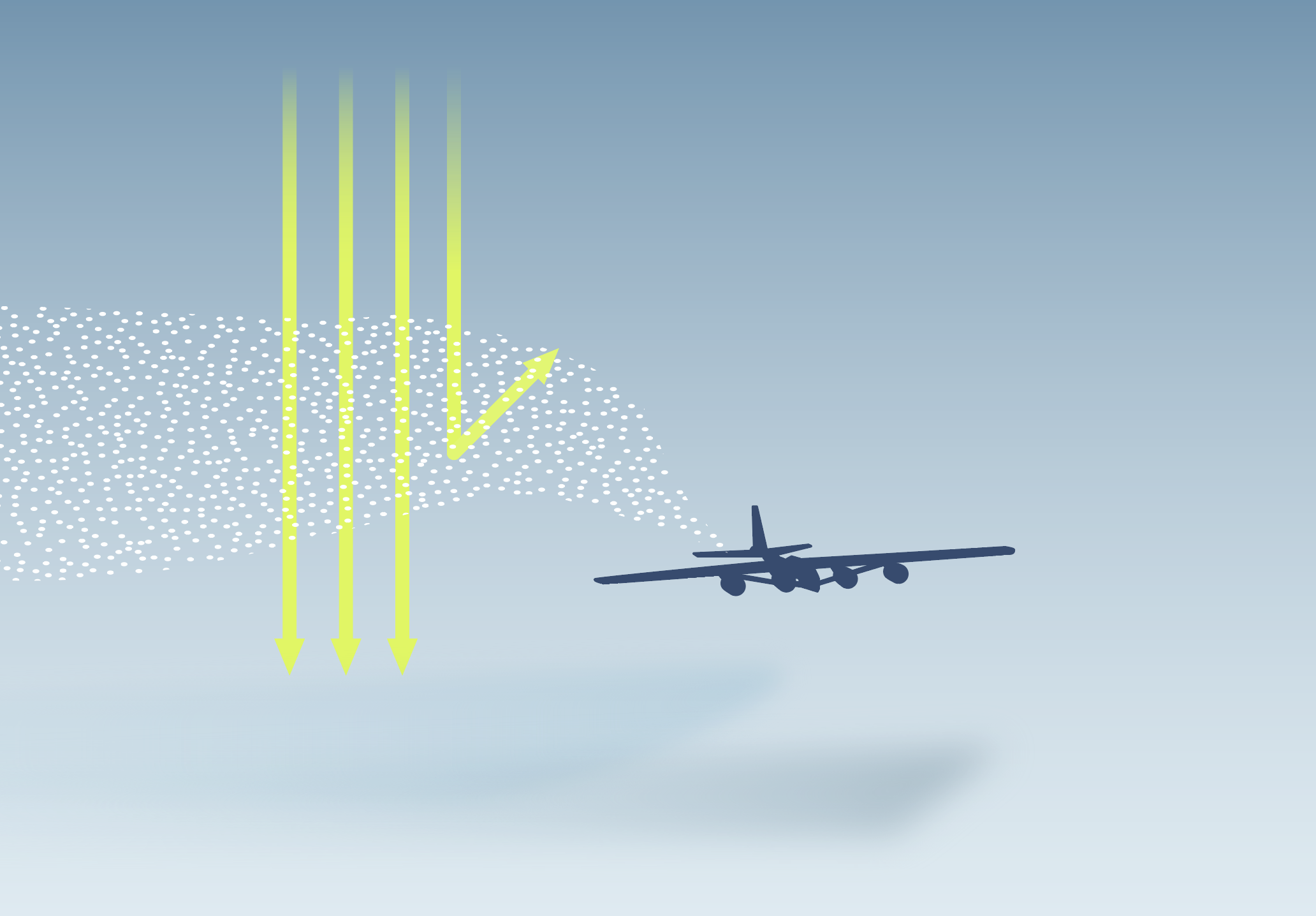
Deploying SAI to cool the planet would involve adding millions of tonnes of tiny particles – known as aerosols – to the stratosphere. The most studied idea is to release SO2 into the stratosphere, which would react to form tiny sulphate aerosol particles.1 However, SO2 emissions are a major contributor to acid rain.2
How significant is the threat from acid rain, and could sulphate-based SAI affect it?
Acid rain formation and impacts
Acid rain refers to any form of precipitation that is more acidic3 than unpolluted rainwater. However, unpolluted rainwater is slightly acidic (pH around 5.6) because water and carbon dioxide in the atmosphere naturally react to form weak carbonic acid.4
When water, oxygen, and other chemicals react with certain pollutants in the atmosphere, primarily SO2 and nitrogen oxides, acid rain is formed. The main source of SO2 emissions is the burning of fossil fuels with sulphur impurities.
Acid rain harms ecosystems in several ways. It can change the acidity of soils and inland water systems making them no longer suitable for some organisms. It can also dissolve nutrients in soils, weakening trees and other plants, and release aluminium from soil, which can be carried to lakes and streams, harming plants and aquatic animals.
The worst impacts of acid rain are concentrated near heavily industrialised regions due to the intense burning of fossil fuels, though air pollution regulations can help to mitigate this. While acid rain itself is not particularly harmful to humans, the SO2 and other pollutants emitted also contribute to poor air quality, posing serious risks to human health.
The effort to clean up acid rain
As the consequences of air pollution on health and the environment became clearer,5 governments began to enact regulations to limit certain pollutants, such as the 1990 Clean Air Act in the United States. As a result, emissions of SO2 and other pollutants have decreased substantially in Europe and North America since the 1970s. Efforts to decrease these emissions in Asia are also starting to yield results.
These efforts have decreased acid rain substantially and more progress is expected in the future. SO2 emissions are projected to decrease by more than 75% globally by the end of this century relative to 2020 even under a high emissions scenario.2
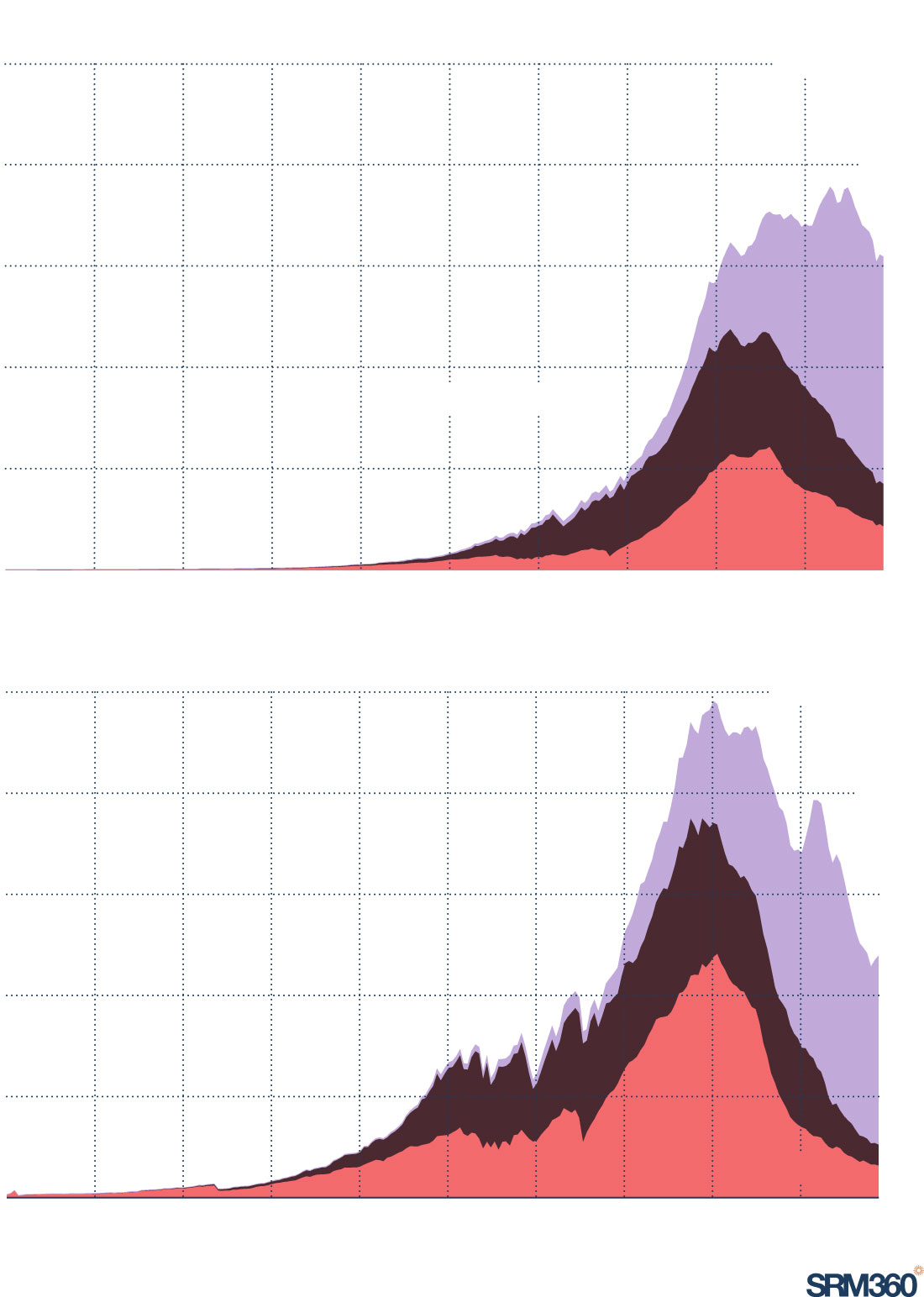
Acid rain is declining after pollution cuts
Emissions of nitrogen oxides and sulphur dioxide are the leading contributors to acid rain. Emissions of both peaked around the 1980s in Europe and North America and have also peaked in Asia in recent years.

NITROGEN OXIDEs (NOx) emissions
125m tonnes
100
75
Asia
50
North America
25
Europe
0
1800
1850
1900
1950
2022
Sulphur dioxide (SO2) emissions
125m tonnes
Asia
100
75
50
North America
25
Europe
0
1850
1900
2022
1800
1950
Source: Community Emissions Data System (CEDS) via Our World in Data
NITROGEN OXIDEs (NOx) emissions
125m tonnes
100
75
Asia
50
North America
25
Europe
0
1950
1975
2022
1750
1850
1900
Sulphur dioxide (SO2) emissions
125m tonnes
Asia
100
75
North America
50
25
Europe
0
1750
1850
1900
1950
1975
2022
Source: Community Emissions Data System (CEDS) via Our World in Data
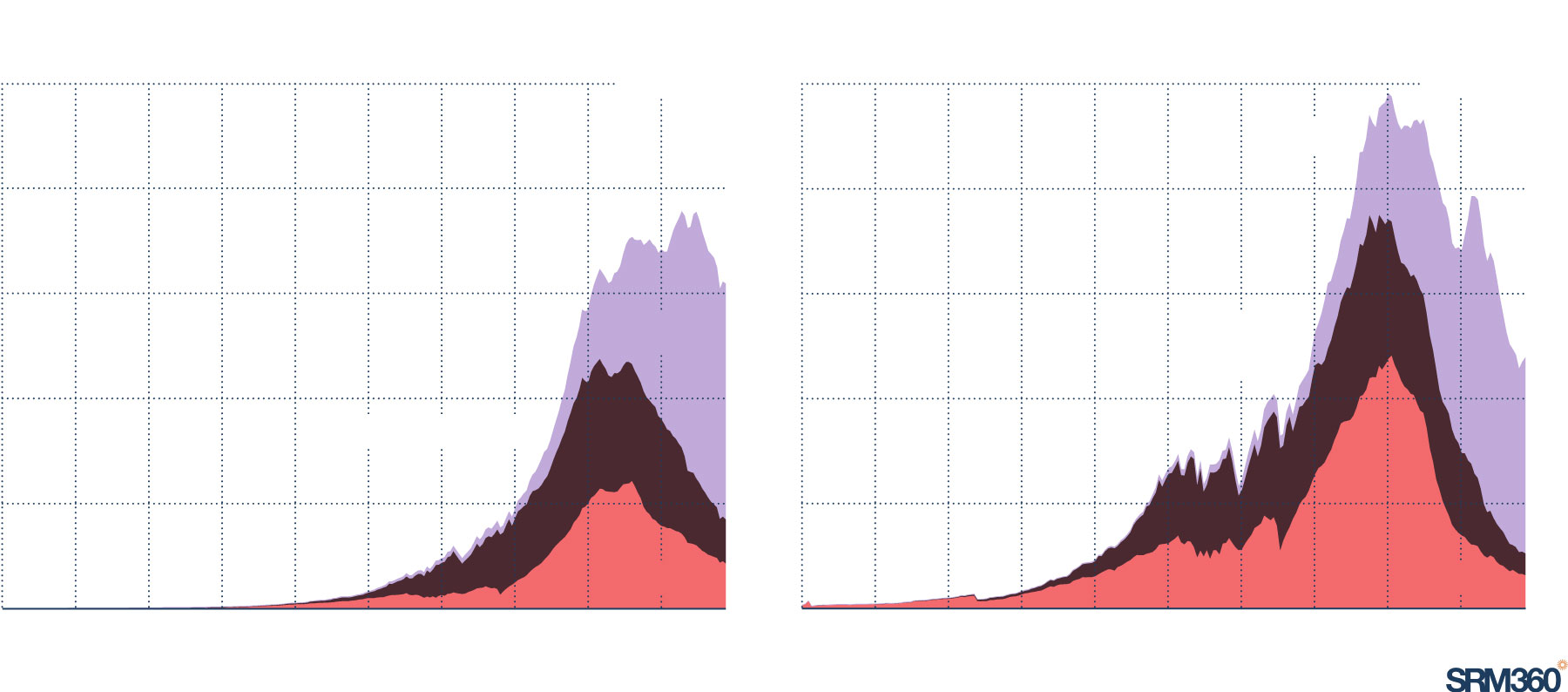
Sulphur dioxide (SO2) emissions
NITROGEN OXIDEs (NOx) emissions
125m tonnes
125m tonnes
Asia
100
100
75
75
Asia
North America
50
50
North America
25
25
Europe
Europe
0
0
1750
1850
1900
1950
1975
2022
1750
1850
1900
1950
1975
2022
Source: Community Emissions Data System (CEDS) via Our World in Data
Acid rain from SAI
SAI would cool the planet by adding aerosol particles in the stratosphere to reflect a small amount sunlight back to space. Studies often focus on using SO2, which reacts to form sulphate aerosols.1
The aerosol particles have a lifetime of around one to two years in the stratosphere,6 and would have to be released continuously to maintain the cooling effect. All the material released for SAI would eventually fall back to the Earth’s surface.2
Under a moderate emissions scenario, global sulphate deposition would fall by roughly 60% by the end of this century according to one study.2 If SAI were used to keep temperatures at 2020 levels under this scenario, offsetting more than 2°C of warming, global sulphate deposition would only fall by roughly 40%.2
While the total amount of acid rain in this SAI scenario would be lower than current levels, its distribution would be different. The aerosols from SAI would eventually settle at high latitudes, so SAI would add very little to acid rain in the tropics and subtropics.2
If SAI were deployed, most areas currently and historically affected by acid rain would still see less in the future, but acid rain could increase in regions that have seen little acid rain to date, such as the Pacific Northwest and Southern Greenland.2 However, for most ecosystems, these increases are unlikely to be substantial enough to cause harm.7
Although most SAI research focuses on sulphate particles, some scientists have suggested using other particles such as calcite or alumina, which would avoid some side effects like acid rain.8 However, much less is known about the effects of these alternative aerosols in the stratosphere compared to sulphates.
Open questions
- How significant would the biodiversity impacts of the acid rain from SAI with sulphates be relative to the impacts of climate change?
- How rapidly will countries cut their emissions of pollutants that cause acid rain, like SO2, in the future?
- How would alternative aerosols like calcite or alumina change the chemistry of the stratosphere?
Endnotes
- Smith W, Wagner G. (2018). Stratospheric aerosol injection tactics and costs in the first 15 years of deployment. Environmental Research Letters. 13(12):124001. https://doi.org/10.1088/1748-9326/aae98d
- Visioni D, Slessarev E, Macmartin DG, et al. (2020). What goes up must come down: Impacts of deposition in a sulfate geoengineering scenario. Environmental Research Letters; 15. https://doi.org/10.1088/1748-9326/ab94eb
- Acidity is measured on the pH scale, which goes from 0 to 14. A pH of 7 is neutral. A pH above 7 is alkaline, while a pH below 7 is acidic. Since the pH scale is logarithmic, stomach acid (pH around 1) is 10 times more acidic than lemon juice (pH around 2) and 10,000 times more acidic than coffee (pH around 5).
- Ocean acidification results primarily from reactions between atmospheric carbon dioxide and ocean water. Acid rain only makes a minor contribution to ocean acidification.9
- Fowler D, Brimblecombe P, Burrows J, et al. (2020). A chronology of global air quality: The development of global air pollution. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences; 378. https://doi.org/10.1098/rsta.2019.0314
- Crutzen PJ. (2006). Albedo enhancement by stratospheric sulfur injections: a contribution to resolve a policy dilemma? Climatic change. 77(3-4):211. https://doi.org/10.1007/s10584-006-9101-y
- Kravitz B, Robock A, Oman L, et al. (2009). Sulfuric acid deposition from stratospheric geoengineering with sulfate aerosols. Journal of Geophysical Research: Atmospheres. 114(D14). https://doi.org/10.1029/2009JD011918
- Keith DW, Weisenstein DK, Dykema JA, et al. (2016) Stratospheric solar geoengineering without ozone loss. Proceedings of the National academy of Sciences. 2016 Dec 27;113(52):14910-4. https://doi.org/10.1073/pnas.1615572113
- Doney SC, Mahowald N, Lima I, et al. (2007). Impact of anthropogenic atmospheric nitrogen and sulfur deposition on ocean acidification and the inorganic carbon system. Proceedings of the National Academy of Sciences. 104(37):14580-5. https://doi.org/10.1073/pnas.0702218104